Color of Light Emitted by Potassium Chloride
In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts.
Student Sheet
In this practical I will be:
- Observing and recording the findings of the practical
- Providing oral and written explanations of my observations, based on scientific evidence and understanding.
- Comparing and grouping materials on the basis of their observable properties.
Introduction:
A local priest has claimed that he has the ability to directly talk to the gods, such as Anubis and Osiris. As an ancient Egyptian science-artist you are highly sceptical of his claims. After going to see this priest it turns out that he is turning flames different colours by throwing different ground minerals on to the flame. Obviously it isn't the gods' doing but you are intrigued as to what is happening. You decide to investigate further…
Equipment:
(Wear safety glasses and tie back long hair)
Method 1:
- Saturated calcium ethanoate solution (must be saturated)
- Ethanol (IDA)
- Lithium chloride (LiCl) solution in a spray bottle; 1 spatula amount in 100 cm3 water
- Copper(II) chloride (CuCl2) solution in a spray bottle;1 spatula amount in 100 cm3 water
- Sodium chloride (NaCl) solution in a spray bottle; 1 spatula amount in 100 cm3 water
- 2 heat resistant mats
- 1 spatula
- 1 beaker (250 cm3)
Method 2:
- Lithium chloride (LiCl) solution in a beaker; 1 spatula amount in 100 cm3 water
- Copper(II) chloride (CuCl2) solution in a beaker; 1 spatula amount in 100 cm3 water
- Sodium chloride (NaCl) solution in a beaker; 1 spatula amount in 100 cm3 water
- 1 heat resistant mats
- Bunsen burner
- 12 cm length of nichrome or platinum wire
Method 3:
The following solutions each in a 250 cm3 conical flask:
-
2 M calcium chloride (IRRITANT)
-
1 M copper(II) chloride (IRRITANT)
-
2 M lithium chloride (IRRITANT)
-
2 M potassium chloride (low hazard)
-
1 M strontium chloride (IRRITANT)
-
2 M sodium chloride (low hazard)
Access to:
- Plenty of spills soaked in water overnight.
- Bunsen burners or adjustable commercial blow torch
- Matches
- Dry spills
Method 1:
- Pour 50 cm3 of the saturated calcium ethanoate solution into the 250 cm3 beaker. Carefully add ethanol to the calcium ethanoate.
- Stir until a solid is formed. If no solid is formed add more ethanol.
- Using a spatula carefully lift out the solid and place it on a heat resistant mat.
- Let it stand for a minute to allow it to dry enough to be lit.
- Use a lighted splint to light the solid.
- Spray the flame with the lithium salt solution. Note the colour and record the result.
- Spray with the copper salt solution. Note the colour and record the result.
- Spray with the sodium salt solution. Note the colour and record the result.
- Put the flame out by carefully placing the other heat resistant mat on top of it.
Method 2:
- Take the nichrome or platinum wire and create a small loop at the end by bending the wire.
- Light the Bunsen burner.
- Turn the collar on the Bunsen burner so that you have an invisible or pale blue flame.
- Burn the loop end of the wire to remove any dust.
- Dip the loop into the lithium salt solution.
- Place the wet loop on the edge of the Bunsen flame.
- Observe and record the colour seen.
- Burn the loop end of the wire to remove any lithium salt.
- Dip the loop into the copper salt solution.
- Place the wet loop on the edge of the Bunsen flame.
- Observe and record the colour seen.
- Burn the loop end of the wire to remove any copper salt.
- Dip the loop into the sodium salt solution.
- Place the wet loop on the edge of the Bunsen flame.
- Observe and record the colour seen.
Method 3:
- Put a dry spill into each of the metal salt solutions in conical flasks and leave.
- Use a dry spill to light the Bunsen.
- Take one of the spills from one of the conical flasks containing a metal salt solution.
- Wave your spill over the Bunsen flame and observe its colour. Then extinguish the used spill and dispose of it.
- Record the metal salt solution and the flame colour.
- Repeat steps 2 to 4 for each of the other metal salt solutions you have been provided with.
Theory:
Calcium ethanoate is a very hygroscopic solid. This means it absorbs and coordinates with water very easily. When ethanol is added to a saturated aqueous solution of calcium ethanoate it forms a white gel. This is because the calcium ethanoate is relatively insoluble in ethanol, as opposed to water, so it precipitates as an inflammable solid, a firelighter that burns with a very clear flame so that any colour given to the flame is due to the metal ion in the salt solution.
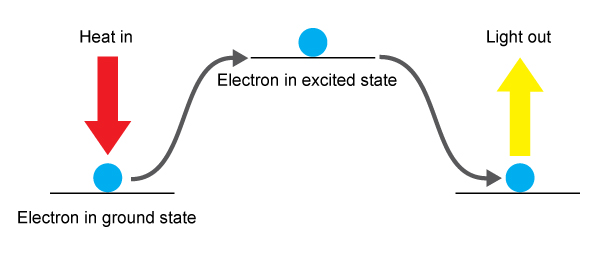
When a metal salt solution is sprayed onto the flame the electrons in the metal are excited and jump from one electron shell level to the next highest shell level. They are said to be excited. They cannot remain there so as they return to the original shell, known as the grounded state the energy gained is lost in the form of light known as emission.
The colour of the light depends upon the metal (lithium(I) gives a magenta red-pink flame, calcium an orange red flame, potassium a lilac flame, strontium a crimson red flame, copper(II) gives a blue or green flame and sodium(I) gives a yellow flame). These colours are also often used in fireworks to give the different colours we see when they burn. Sodium is also used in some street lights and that is why they appear yellow when on.
If the flame is looked at through a spectroscope it will give a characteristic spectrum. This is used in chemistry to analyse a material for type and concentration of atoms. Chemists 'burn' the substance and measure the frequency (colour) of the light given out. This process is called Atomic Emission Spectroscopy.
Teacher and Technician Sheet
In this practical students will:
- Observe and record the findings of the practical
- Provide oral and written explanations of their observations, based on scientific evidence and understanding.
- Compare and group materials on the basis of their observable properties.
Introduction:
This is an old and tested experiment but when dealing with colour and chemistry it would be difficult to leave it out – particularly if spectroscopy is to be considered.
It is possible to create a variety of coloured flames by burning a small amount of different metal salts in a fire. This is the basis of fireworks.
In chemistry terms the fact some metals burn with a characteristic flame colour is important since it allows us to introduce the concept of spectroscopy.
As an introduction fireworks might be a good starting point. A discussion could begin with what it is that makes them spectacular and lead to the types of effects seen in fireworks, especially the colours.
Curriculum range:
This activity is designed for secondary age students but could be used with upper primary pupils. It links with:
- reporting on findings from enquiries, including oral and written explanations, displays or presentations of results and conclusions;
- using straightforward scientific evidence to answer questions or to support their findings;
- comparing and grouping together materials on the basis of their properties;
- building a more systematic understanding of materials by exploring; and comparing the properties of a broad range of materials.
Going further:
Working pairs students can look at the flame colour using a spectroscope which can be a laboratory one or one they build themselves. There are directions to be found by clicking here.
Hazard warnings:
Calcium ethanoate – Low hazard
Ethanol (IDA) – Flammable may be harmful by inhalation, ingestion or skin absorption may act as an irritant.
Lithium chloride – Solid is Acute Toxin Cat 4 (HARMFUL)
Copper(II) chloride – Acute Toxin Cat 4 (HARMFUL) and a SKIN/EYE IRRIRANT (Cat 2) and HAZARDOUSTO THE AQUATIC ENVIRONMENT WITH LONG LASTING EFFECTS (cat 1)
Sodium chloride – No significant risk (Low Hazard)
Potassium chloride – No significant risk (Low Hazard)
Strontium chloride – Can cause SERIOUS EYE DAMAGE (Cat 1) and is a SKIN IRRITANT (Cat 2) and a RESPIRATORY IRRITANT.
Safety goggles and should be worn. Long hair should be tied back and secured when using naked flames in a laboratory.
Avoid permanganates, nitrates and chlorates. These produce harmful by-products when burned.
Equipment for method 1:
- Saturated calcium ethanoate solution (must be saturated)
- Ethanol
- Lithium chloride (LiCl) solution in a spray bottle; 1 spatula amount in 100 cm3 water
- Copper(II) chloride (CuCl2) solution in a spray bottle; 1 spatula amount in 100 cm3 water
- Sodium chloride (NaCl) solution in a spray bottle; 1 spatula amount in 100 cm3 water
- 2 heat resistant mats
- 1 spatula
- 1 beaker (250 cm3)
Equipment for method 2:
- Lithium chloride (LiCl) solution in a beaker; 1 spatula amount in 100 cm3 water
- Copper(II) chloride (CuCl2) solution in a beaker; 1 spatula amount in 100 cm3 water
- Sodium chloride (NaCl) solution in a beaker; 1 spatula amount in 100 cm3 water
- 1 heat resistant mats
- Bunsen burner
- 12 cm length of nichrome or platinum wire
Equipment for method 3:
The following solutions each in a 250 cm3 conical flask:
- 2 M calcium chloride (SKIN/EYE IRRITANT)
- 1 M copper(II) chloride (SKIN/EYE IRRITANT)
- 2 M lithium chloride Low hazard
- 2 M potassium chloride (Low Hazard)
- 1 M strontium chloride (SKIN IRRITANT, EYE DAMAGE)
- 2 M sodium chloride (Low Hazard)
Access to:
- Plenty of spills soaked in water overnight.
- Bunsen burners
- Heatproof mats
- Matches
- Dry spills
Technical notes:
This experiment can be accompanied by the RSC's Flame Colours – a demonstration carried out by the teacher as instructed.
The teacher demonstration is the only time that ethanol should be near a naked flame.
The metal salt solutions can be made and stored in conical flasks stoppered with rubber bungs prior to using.
Some spills are soaked in water to ensure that the flame colour can be observed properly before the spill burns away and reduces the risk of burning to the student.
When preparing for use, the excess water can be squeezed from the spills that have been soaking in water overnight before placing some of them in each of the conical flasks containing the metal salt solutions.
Beakers (or similar) containing water could be provided for the students to use to extinguish their spills.
Results:
Method 3 is very easy to set up and use.
It is safe from Year 7 upwards and the teacher demonstration suggested can accompany it.
The students should be able to observe and record the relevant flame colours and understand the reasons behind this from the accompanying notes.
Cobalt blue glass can be provided if available. The metal salt's flame colour may be observed more easily when the yellow light is absorbed by the blue in the glass.
Lithium – magenta red flame
Calcium - orange red flame
Potassium - lilac flame
Strontium – crimson red flame
Copper – blue or green flame (depends on the copper used)
Sodium - yellow flame
The accompanying notes may need to be adjusted depending upon whether all the method options are provided or not.
Color of Light Emitted by Potassium Chloride
Source: https://edu.rsc.org/resources/flame-tests-using-metal-salts/1875.article